8 Specific Drugs
Antibiotics
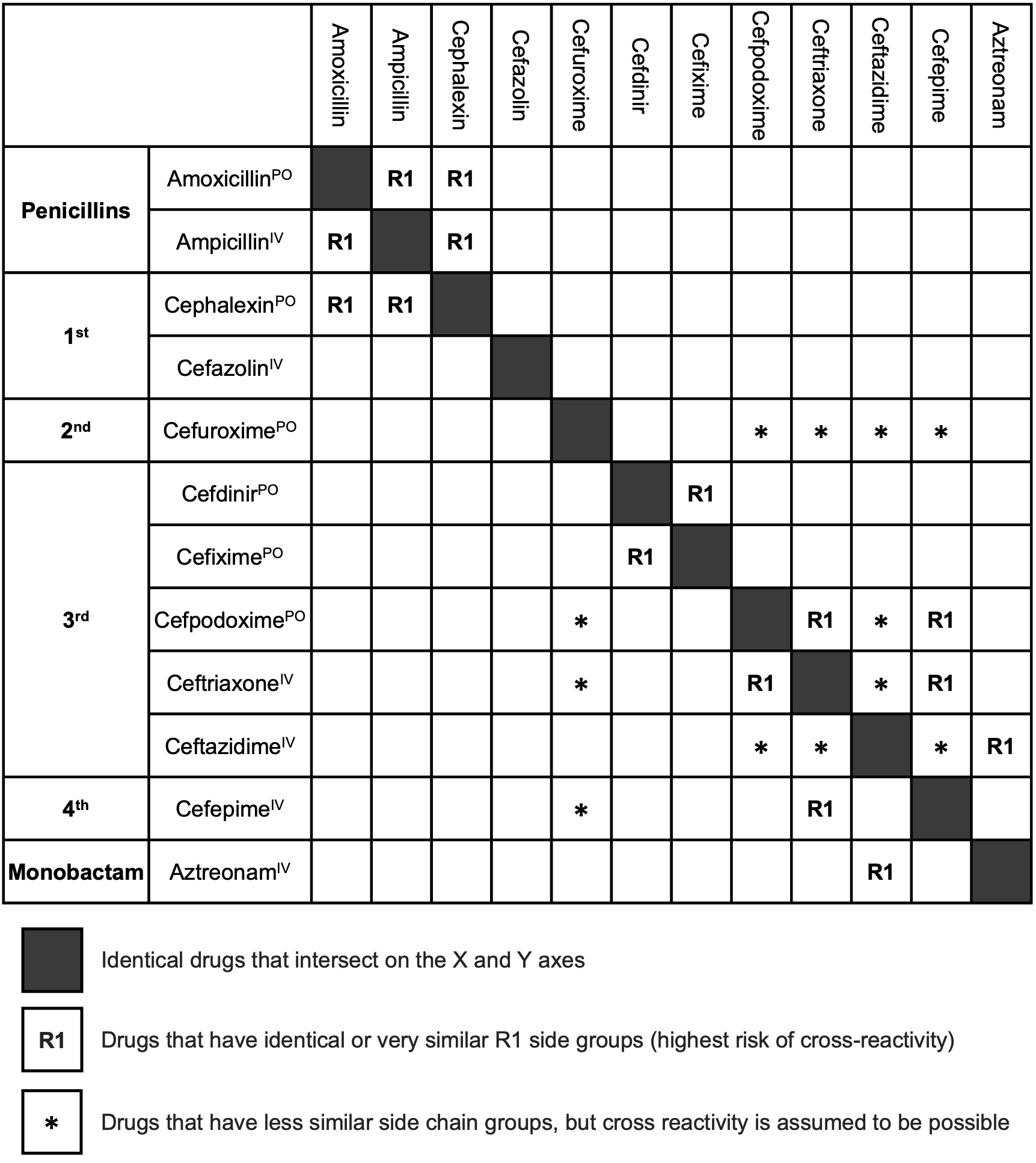
Cephalosporins
Background
Skin Testing
Lack of commercial products and metabolites for skin testing.
Immediate Index agents: cephalexin (55.5%), ceftriaxone (24.9%), and cefazolin (17.5%)
8.9% of pateints had positive skin tests (cefazolin 50% positives)
Time since index reaction, OR 0.71 (95% CI 0.57 - 0.90) for positive skin test. (Stone, Trubiano, and Phillips 2021)
Oral Challenge
100% NPV if reaction > 5 years, isolated urticaria, benign MPE, isolated GI symptoms or other non-allergic and orally administere vs 98.5% NPV (95% CI 95.8%-99.7%) if include IV and PO administered. (Stone, Trubiano, and Phillips 2021)
Cephalexin 250 mg oral challenge with 1.5 hour observation. (Koo et al. 2022)
Fluoroquinolones
Macrolides
Background
Skin Testing
Skin testing for macrolide immediate hypersensitivity has not been shown to be reliable.
Oral Challenge
For patients with a history of immediate hypersensitivity reaction to a macrolide, graded azithromycin challenge can be performed, starting with azithromycin 25 mg followed by 1 hour observation then 250 mg followed by 2 hour observation.
For patients with a history of non-severe delayed hypersensitivity reaction to a macrolide, single dose azithromycin 250 mg challenge with 2 hour observation can be performed. Patients should be instructed to report any other delayed symptoms, which may occur up to 24 to 48 hours after the challenge dose.
Penicillins
Background
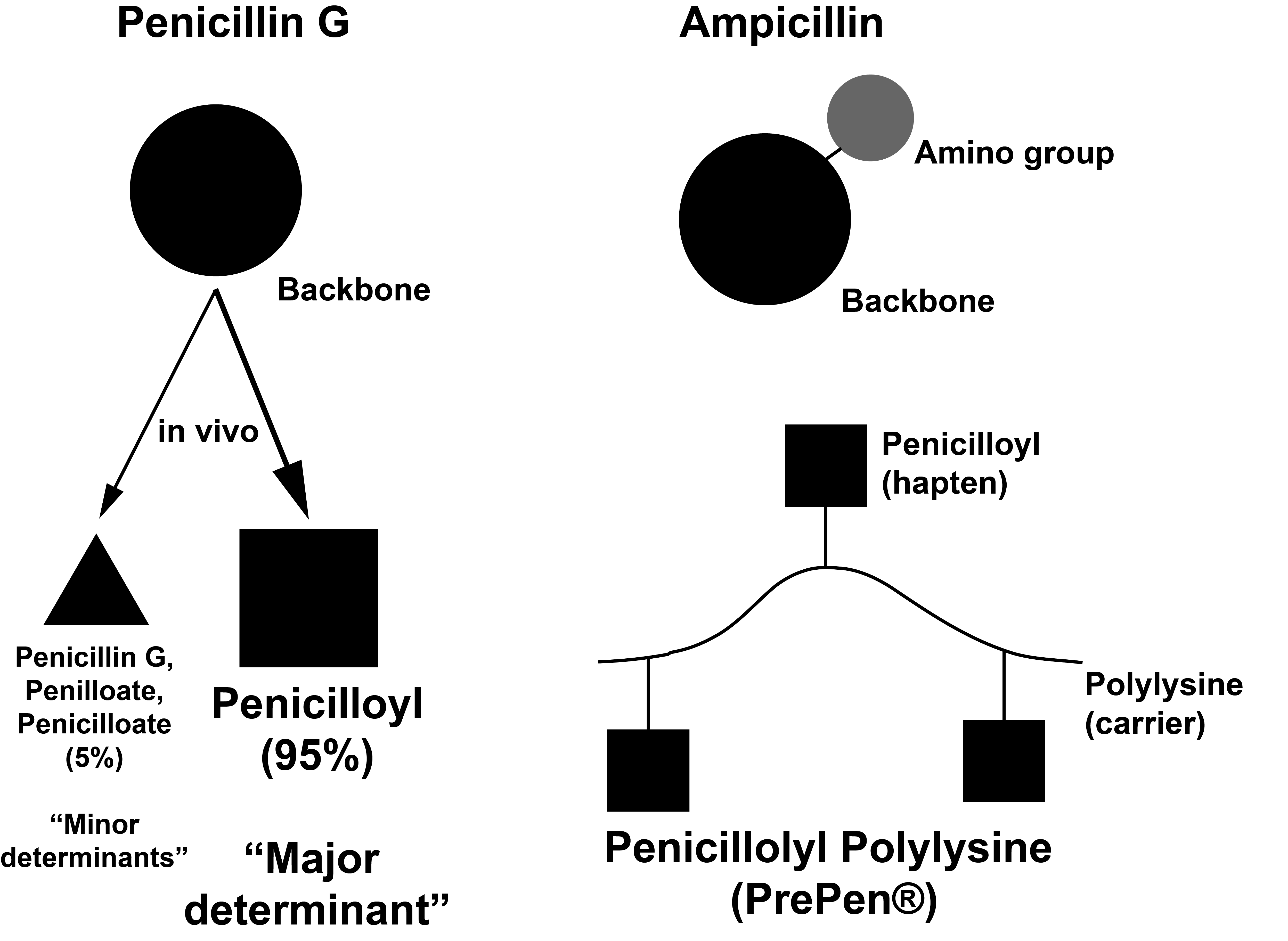
Skin Testing
| Reagent | Description |
|---|---|
| Penicilloyl polylysine (PrePen®) |
|
| Minor derterminant mixture (MDM) |
|
| Penicillin G |
|
| Ampicillin |
|
Selective IgE-mediated reactions to aminopenicillins are rare in North America (e.g. 3-5% in the United States) versus 25-50% of skin test positive patients in Europe.
The polylysine carrier has ~40 lysine repeats.
Oral Penicillin = Penicillin VK IM/IV Penicillin = Penicillin G Amino group = -NH3
Aminopenicillins = Amoxicillin, Ampicillin Aminocephalosporins = Cephalexin, Cefadroxil, Cefprozil and Cefaclor
References:
Chapter 77 Middleton’s Drug Allergy
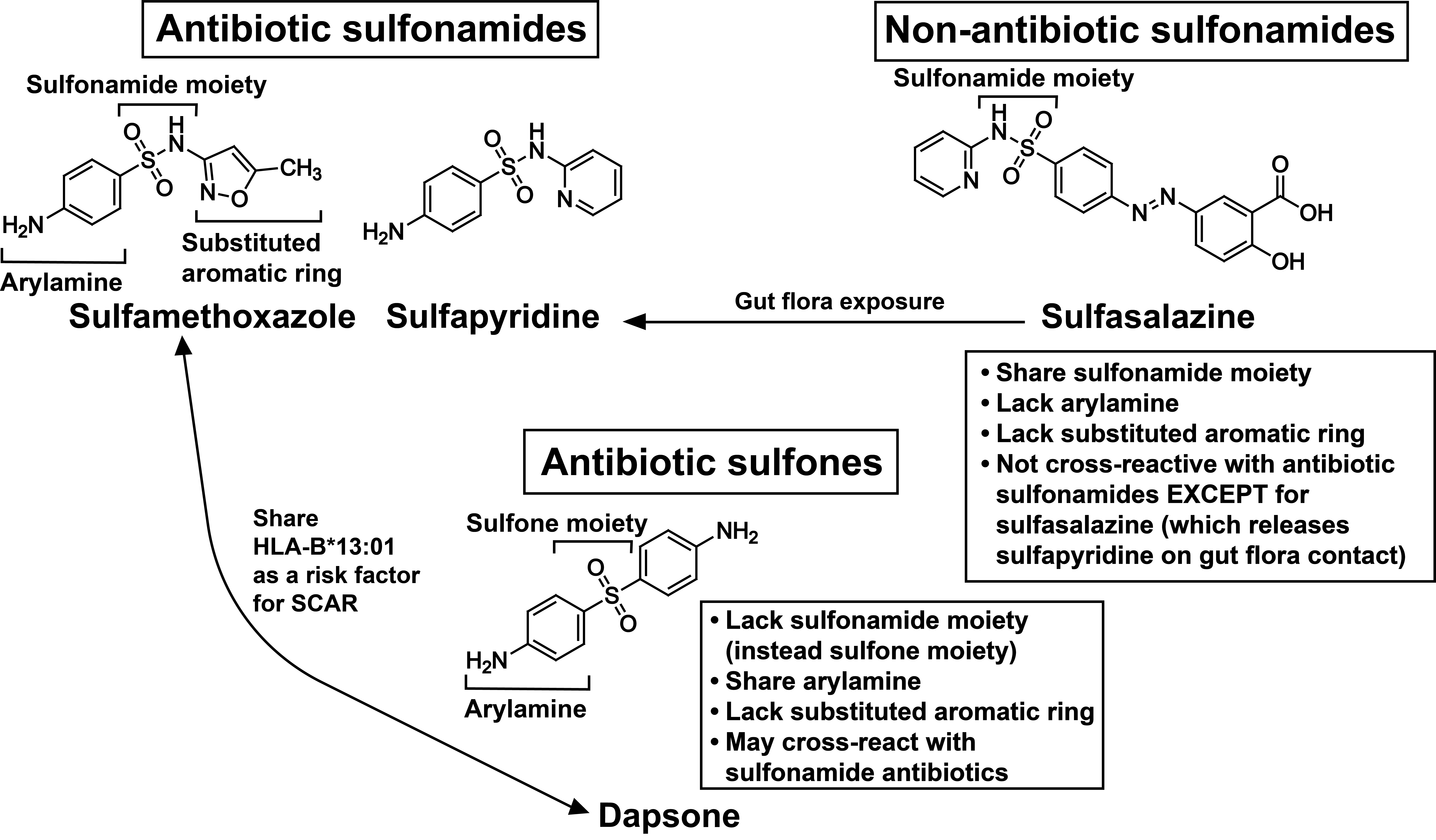
Sulfa Antibiotics
Background
Vancomycin
Antiepileptic Drugs
Background
| Aromatic AEDs | Non-aromatic AEDs |
|---|---|
|
|
Reference
Mani R, Monteleone C, Schalock PC, Truong T, Zhang XB, Wagner ML. Rashes and other hypersensitivity reactions associated with antiepileptic drugs: A review of current literature. Seizure. 2019 Oct;71:270-278. doi: 10.1016/j.seizure.2019.07.015. Epub 2019 Jul 24. PMID: 31491658.
Antihistamines
References
Buproprion
Gadolinium-Based Contrast Agents
Background
| Class | Generic Name (Brand Name) | Immediate Reactions per 10,000 Administrations |
|---|---|---|
| Linear, nonionic | Gadodiamide (Omniscan) | 1.5 |
| Linear, ionic | Gadopentetate (Magnevist), Gadoxetate (Eovist), Gadobenate (MultiHance), Gadofosveset (Ablavar) | 8.3 |
| Macrocyclic | Gadobutrol (Gadovist), Gadoterate (Dotarem), Gadoteridol (ProHance) | 16 |
References
Iron
Background
| Generic name | Iron gluconate | Iron Sucrose | LMWID | Ferric carboxymaltose | Iron isomaltoside | Ferumoxytol |
|---|---|---|---|---|---|---|
| Brand name | Ferrlecit | Venofer | INFeD | Injectafer | Monofer | FeraHeme |
| Molecular weight (kD) | 289-440 | 30-60 | 165 | 150 | 150 | 750 |
| Labile iron (% injected dose) | 3.3 | 3.5 | 2 | 0.6 | 1 | 0.8 |
Immediate Hypersensitivity Reactions
Minor Infusion Reactions
Skin Testing
Management
References
Gómez-Ramírez S, Shander A, Spahn DR, et al. Prevention and management of acute reactions to intravenous iron in surgical patients. Blood Transfusion. Published online April 10, 2019. doi:10.2450/2018.0156-18
Muñoz M, Gómez-Ramírez S, Bhandari S. The safety of available treatment options for iron-deficiency anemia. Expert Opin Drug Saf 2018; 17: 149-59.
Latex
Background
NRL = natural rubber latex > 40,000 products with NRL
Hard rubber latex (heat vulcanized at high temp) Less latex antigen (most allergen denatured at high heat) Medical Consumer Gloves, balloons, condoms
Soft rubber latex (dipping process low heat vulcanization; 10-12% latex products made this way) More latex antigen (less allergen denatured at low heat) Bungs, stoppers Medical Consumer rubber car tires
Skin Testing
- Standardized latex for skin testing is not available in the US.
-
95% Sensitivity (false negative can occur with non-standardized extracts), 100% specificity
Specific IgE Testing
~70-80% sensitivity, 95% specificity
Management
In US, medical devices are required to be labelled with warning if contain NRL.
Latex avoidance if subsequent serologic testing negative; however, negative latex IgE susggestive that inadvertant exposure risk is minimal.
Local Anesthetics
Background
| Amides | Esters |
|---|---|
|
|
Skin Testing
LA preparations without epinephrine should be used for skin testing because the vasoconstricting properties of epineprhine may mask a positive test.
Management
If skin testing is negative to lidocaine, a subcutaneous lidocaine 1% with 1.5 mL (15 mg) can be performed followed by a 1.5 hour observation.
Nonsteroidal Anti-Inflammatory Drugs
Background
Management
For non-aspirin exacerbated respiratory disease (AERD) NSAID hypersensitivity, a two-step outpatient NSAID oral challenge has been shown to be a safe and effective approach.
Oral Challenge
| NSAID | Step 1 Dose (60 minute observation) |
Step 2 Dose (120 minute observation) |
|---|---|---|
| Aspirin | 40.5 mg | 325 mg |
| Ibuprofen | 50 mg | 500 mg |
| Naproxen | 60 mg | 600 mg |
Because of the potential future need for higher dose of aspirin for management of acute coronary syndrome, the second dose for aspirin oral challenge is 325 mg instead of 81 mg.
Of the 262 NSAID challenges performed, over 85% had negative challenges. In addition, 76% of patients included in the study reported a history of urticaria, angioedema, or both (Li et al. 2022). For patients experiencing a positive challenge, 45% had their reaction within 3 hours of NSAID ingestion
Radiocontrast
Background
Infusion Reactions
These are also referred to as “toxic” or “chemotoxic” reactions. Characteristic symptoms include transient warmth/flushing, nausea/vomiting, chest pain, metallic taste, hypertension, and/or vasogal signs.
Immediate Hypersensitivity Reactions
The most common immediate hypersensitivity reaction to radiocontrast is mild urticaria and pruritus, occuring in ~0.9% - 3.1% of patients receiving radiocontrast. Anaphylaxis occurs in 0.02% - 0.04% of patients. Of immediate hypersensitivity reactions, 70% occur within 5 minutes of radiocontrast injection and 96% of severe reactions occur within 20 minutes.
Delayed Hypersensitivity Reactions
The most common delayed hypersensitivity reaction to radiocontrast is a maculopapular exanthem–occurring in 1 to 3% of patients.
Skin Testing
Skin prick testing to the culprit and other radiocontrasts followed by intradermal testing–if skin prick testing is negative–can be useful for identifying an alternative radiocontrast agent. A skin test negative radiocontrast alternative has a 95% NPV.
Intravenous Challenge
Intravenous challenge can be performed to radiocontrast with various protocols–such as 1 mL, 5 mL, 15, mL, and 50 mL (cumulative 71 mL) at 60 minute intervals.
Management
The culprit radiocontrast should be avoided, and an alternative radiocontrast should be used, guided by negative skin testing if available. Other measures to decrease risk of recurrent radiocontrast reaction include: lowering the radiocontrast dose, decreasing the injection speed, and pre-treatment with non-sedating, second generation antihistamines and/or corticosteroids.
Topicals
Antibiotics
The most common topical antibiotics which cause ACD are those found in triple antibiotic ointment—neomycin, polymyxin B, and bacitracin (Choi et al. 2021).
Of patients with neomycin ACD, 50% will cross-react with other aminoglycosides such as gentamicin.
Anesthetics
The most common topical anesthetics which cause ACD are lidocaine and benzocaine.
While patch testing can confirm ACD due to a local anesthetic, not all patients will necessarily develop an allergic reaction to the same anesthetic if used intradermally or subcutaneous—which is often done for dental and dermatologic procedures.
Corticosteroids
Most patients with ACD secondary to corticosteroids have a history of atopy. Because corticosteroids are often not considered initially as a possible cause of ACD, there might be increased use and worsening of a patient’s dermatitis.
ACD due to corticosteroids may produce an “edge effect” or “doughnut-type” reaction–due to the anti-inflammatory effect of the higher concentration of the corticosteroid in the central area compared to the periphery.
If a patient has ACD due to a topical corticosteroid, you should consider propylene glycol as a potential cause—an excipient found in various topical corticosteroids and one of the top 4 causes of ACD due to drugs.
Propylene Glycol
Propylene glycol—an excipient—may be utilized in topical drugs as a softening agent, solvent, moisturizer, or preservative.
Of patients with propylene glycol ACD, 80% have a history of atopic dermatitis.
Propylene glycol is present in various topical emolients, corticosteroids, and calcineurin inhibitors (Tran and Reeder 2020).
| Topical | Propylene glycol-containing | Propylene glycol-free |
|---|---|---|
| Emolients | ||
| Corticosteroids |
|
|
| Calcineurin inhibitors |
|
|
Eye Drops
The eyelids are more susceptible to ACD compared to other facial areas—owing to their thin skin 0.55 mm compared to 2 mm, respectively (Amin and Belsito 2006). Therefore, the eyelids may be the only affected area by a drug that comes in contact with the face.
ACD due to eye drops is primarily caused by anti-microbial preservatives rather than the primary drug. Benzylalkonium chloride is the most commonly implicated preservative in patients with history of eye drop reactions (Dear, Palmer, and Nixon 2021).
| Category | Benzyalkonium chloride-containing | Benyzlalkonium chloride-free |
|---|---|---|
| Antibiotic |
|
|
| Corticosteroid |
|
|
| NSAID |
|
|
| Gluacoma |
|
|
References:
https://eyewiki.org/Preservatives_in_Topical_Ophthalmic_Medications#Benzalkonium_chloride_(BAK)
Vaccines
Background
Skin Testing
Management
| Reaction Type | Perform Skin Testing? | Vaccine Administration by Skin Testing Result (if applicable) |
|---|---|---|
| Delayed reaction (e.g. late-onset urticaria, local arm swelling) | No | Subsequent dose in usual manner |
| Immediate reaction without IgE-mediated reaction features (e.g. tingling, flushing, palpitation, sensation of throat closure) | No | Subsequent dose in usual manner but observe for 30 minutes |
| Immediate reaction with IgE-mediated reaction features (e.g. urticaria, angioedema, wheezing) | Yes | Negative: Usual manner 30 min obs Positive: Split dose |
| Anaphylaxis | Yes | Negative: Split dose Positive: Graded dose |
| Step | Volume (mL) of full-dose full-strength for 0.5 mL vaccine dose | Dilution | Percent of full-dose full-strength for 0.5 mL vaccine dose | Observation |
|---|---|---|---|---|
| 1 | 0.05 | Full-strength | 10% | 30 minutes |
| 2 | 0.45 | Full-strength | 90% | 30 minutes |
| Step | Volume (mL) | Dilution | Percent of full-dose full-strength | Observation |
|---|---|---|---|---|
| 1 | 0.05 | 1:10 | N/A | 15 minutes |
| 2 | 0.05 | Full-strength | 10% | 15 minutes |
| 3 | 0.1 | Full-strength | 20% | 15 minutes |
| 4 | 0.15 | Full-strength | 30% | 15 minutes |
| 5 | 0.2 | Full-strength | 40% | 15 minutes |